RGD imaging
The microenvironment around tumors is made up of many different cell types that influence tumor growth and metastasis. Angiogenesis, the growth of new blood vessels from preexisting blood vessels, is required for tumors to grow beyond 2-3 mm in diameter. One of the first phases in angiogenesis is the activation of endothelial cells, resulting in the expression of αvβ3-integrin on the cell surface of endothelial cells. αvβ3-integrin has the ability to interact with the extracellular matrix (ECM) proteins such as collagen, vitronectin and fibronectin through the amino acid Arg-Gly-Asp (RGD) sequence. This interaction is crucial in cell migration and survival of cells during angiogenesis. αvβ3-integrin also plays an important role in tumor progression, invasive growth and metastasis.
We developed a radiotracer consisting of two cyclic RGD-peptides that can be labeled with radionuclides (In-111, Ga-68, a.o.) to allow non-invasive visualization of angiogenesis using SPECT/CT and PET/CT. The potential of radiolabeled RGD peptides to image αvβ3-integrin-expressing cells in vivo were assessed in preclinical models. We used patient-derived head and neck tumor xenografts in mice and found that integrin αvβ3 in these tumors is expressed on angiogenic blood vessels and not present on tumor cells themselves.
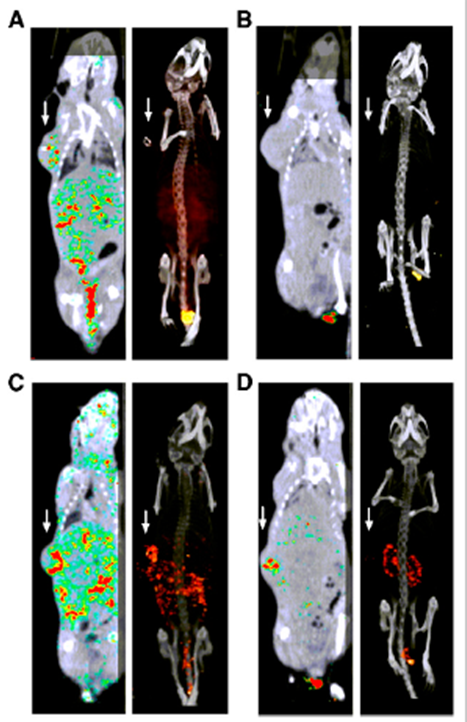
Figure 1: Fused SPECT/CT scans (1 hour post injection) of mice with subcutaneous head and neck tumor xenografts on right flank (white arrows). A and C: mice injected with 111In-DOTA-RGD2. B and D: mice injected with 111In-DOTA-RGD2 and an excess of unlabeled RGD peptide to block the αvβ3-integrin. These scans show that the tracer can visualize angiogenesis.
In another preclinical experiment we determined whether our RGD-based radiotracer could monitor the response of head and neck tumors to various forms of therapy (anti-angiogenic therapy, radiotherapy, or a combination of those two). This preclinical experiment provided evidence that radiolabeled RGD-peptides can be used as an angiogenesis-specific tracer and that it might be a useful tool to monitor responses after therapy.
We currently perform a prospective clinical study in which 25 patients, diagnosed with Head and Neck Squamous Cell Carcinoma (HNSCC) of at least 3 cm and scheduled for surgical resection, are included. In this clinical trial we aim to validate the feasibility and safety of 68Ga-DOTA-(RGD)2 PET/CT as a non-invasive method to assess tumor angiogenesis in HNSCC patients. If successful, 68Ga-DOTA-(RGD)2 PET/CT could, after further clinical investigation, be used to non-invasively monitor the level of angiogenesis in HNSCC patients treated with (chemo)radiotherapy.
Collaborations
- Department of Oral and Maxillofacial Surgery
- ENT department (Ear, Nose and Throat)
- Department of Medical Oncology
People
funding
ZonMw - Programma Translationeel Onderzoek; nr. 95104005; Projecttitle: Imaging with radiolabeled dimeric RGD peptides to monitor response to radiotherapy in head and neck cancer patients.
publications
- S. Terry, K. Abiraj, J. Lok, D. Gerrits, G. Franssen, W. Oyen and O. Boerman. "Can 111In-RGD2 Monitor Response to Therapy in Head and Neck Tumor Xenografts?", 2014. Abstract/PDF DOI PMID
- S. Terry, K. Abiraj, C. Frielink, L. van Dijk, J. Bussink, W. Oyen and O. Boerman. "Imaging Integrin αvβ3 on Blood Vessels with 111In-RGD2 in Head and Neck Tumor Xenografts", 2014. Abstract/PDF DOI PMID
- I. Dijkgraaf, S. Terry, W. McBride, D. Goldenberg, P. Laverman, G. Franssen, W. Oyen and O. Boerman. "Imaging integrin alpha-v-beta-3 expression in tumors with an 18F-labeled dimeric RGD peptide", 2013. Abstract DOI PMID
- I. Dijkgraaf, C. Yim, G. Franssen, R. Schuit, G. Luurtsema, S. Liu, W. Oyen and O. Boerman. "PET imaging of $alpha$v$beta$₃ integrin expression in tumours with �?��?�Ga-labelled mono-, di- and tetrameric RGD peptides", 2011. Abstract DOI PMID




