Antibodies imaging for immune check point inhibitors
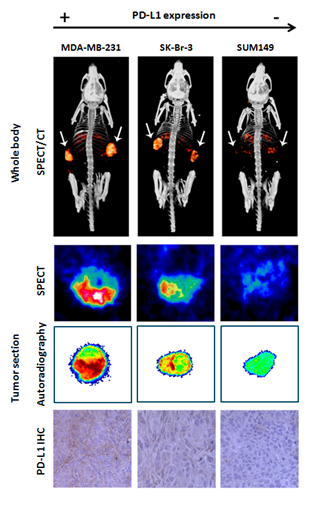
Our immune system is capable of recognizing and eliminating cancer cells. This process is tightly regulated by immune checkpoints and their ligands. A major inhibitory immune checkpoint is programmed death-1 (PD-1), which is expressed by T-cells. Its ligand PD-L1 is expressed by tumor cells and antigen presenting cells (APCs). By upregulating PD-L1, tumor cells inactivate T-cells and escape immune recognition and attack. Clinical trials with anti-PD-1/PD-L1 immune checkpoint inhibitors (ICI) have shown durable (≥ 1 year) antitumor activities in 15-20% of cancer patients. This shows that ICI significantly improve the outcome for a subgroup of patients. However, a large number of non-responding patients is unnecessarily exposed to an expensive, ineffective treatment and its associated side effects. To use these expensive drugs more effectively, there is an urgent need for a biomarker to accurately predict treatment response. Tumor that express high levels of PD-L1 and PD-1 are most likely to respond to ICI. Therefore, we develop novel radiotracers to non-invasively image tumor PD-1 and PD-L1 expression by PET and SPECT. Recently, we have shown that it is feasible to distuingish high, moderate, and low PD-L1 expressing tumors by SPECT/CT imaging, using radiolabeled anti-PD-L1 antibodies.
Collaborations
- Willemijn Hobo
- Harry Dolstra
- Laboratory Medicine – Laboratory of Hematology, Radboud University Medical Center, Nijmegen, The Netherlands
People
Publications
- S. Lütje, S. Heskamp, A. Cornelissen, T. Poeppel, S. van den Broek, S. Rosenbaum-Krumme, A. Bockisch, M. Gotthardt, M. Rijpkema and O. Boerman. "PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status", 2015.
- S. Heskamp, W. Hobo, J. Molkenboer-Kuenen, D. Olive, W. Oyen, H. Dolstra and O. Boerman. "Non-invasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies", 2015. Abstract/PDF DOI PMID
- K. Aben, S. Heskamp, M. Janssen-Heijnen, E. Koldewijn, C. van Herpen, L. Kiemeney, E. Oosterwijk and D. van Spronsen. "Better survival in patients with metastasised kidney cancer after nephrectomy: a population-based study in the Netherlands", 2011.