NucMed
Research in the Nucmed group focuses on the development of novel tracers to image disease processes using radionuclide and optical imaging techniques. The main areas of interest are cancer, diabetes, rheumatoid arthritis, and neurological disorders. In addition, for certain applications, these tracers are used as therapeutic agents as well. Our translational research lines start with tracer development, followed by preclinical characterization in animal models and finally clinical studies are carried out in patients. Please click here for an overview of all projects.
Highlight
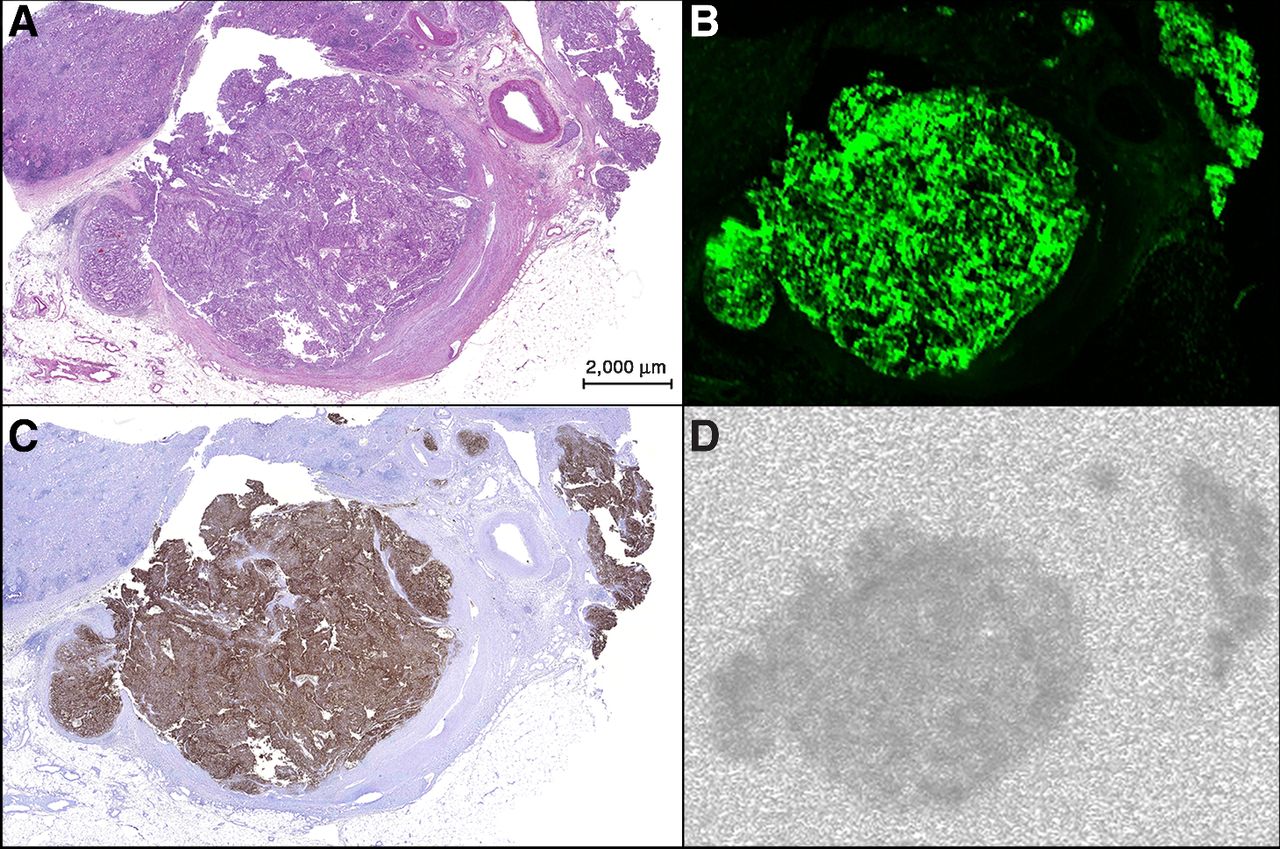 Tissue section of tumor thrombus in the renal vein and several small veins showing the overlap between the fluorescent and radioactive signals and CAIX expression in tumor tissue. A, H&E staining. B, fluorescence image (Odyssey). C, CAIX staining with DAB of CAIX-expressing tumor tissue. D, autoradiography.
Tissue section of tumor thrombus in the renal vein and several small veins showing the overlap between the fluorescent and radioactive signals and CAIX expression in tumor tissue. A, H&E staining. B, fluorescence image (Odyssey). C, CAIX staining with DAB of CAIX-expressing tumor tissue. D, autoradiography.
Marlène C.H. Hekman et al. published her work on targeted dual-modality imaging in Clinical Cancer Research:
Targeted dual-modality imaging in renal cell carcinoma: An ex vivo kidney perfusion study
Purpose: Antibodies labeled with both a near-infrared fluorescent dye and a radionuclide can be used for tumor-targeted intraoperative dual-modality imaging. Girentuximab is a chimeric monoclonal antibody against carbonic anhydrase IX (CAIX), an antigen expressed in 95% of clear cell renal cell carcinoma (ccRCC). This study aimed to assess the feasibility of targeted dual-modality imaging with 111In-girentuximab-IRDye800CW using ex vivo perfusion of human tumorous kidneys.
Experimental Design: Seven radical nephrectomy specimens from patients with ccRCC were perfused during 11 to 15 hours with dual-labeled girentuximab and subsequently rinsed during 2.5 to 4 hours with Ringer's Lactate solution. Then, dual-modality imaging was performed on a 5- to 10-mm-thick lamella of the kidney. Fluorescence imaging was performed with a clinical fluorescence camera set-up as applied during image-guided surgery. The distribution of Indium-111 in the slice of tumor tissue was visualized by autoradiography. In two perfusions, an additional dual-labeled control antibody was added to demonstrate specific accumulation of dual-labeled girentuximab in CAIX-expressing tumor tissue.
Results: Both radionuclide and fluorescence imaging clearly visualized uptake in tumor tissue and tumor-to-normal tissue borders, as confirmed (immuno)histochemically and by gamma counting. Maximum uptake of girentuximab in tumor tissue was 0.33% of the injected dose per gram (mean, 0.12 %ID/g; range, 0.01–0.33 %ID/g), whereas maximum uptake in the normal kidney tissue was 0.04 %ID/g (mean, 0.02 %ID/g; range, 0.00– 0.04 %ID/g).
Conclusions: Dual-labeled girentuximab accumulated specifically in ccRCC tissue, indicating the feasibility of dual-modality imaging to detect ccRCC. A clinical study to evaluate intraoperative dual-modality imaging in patients with ccRCC has been initiated.
To read the full article click here.